Mesenchymal Stem Cells (MSCs): Comprehensive Statistics, Findings, and Research Insights
Written by Dr. David Greene, MD, PhD, MBA on July 30, 2025
The US Leader in Stem Cell Therapy, Now in Mexico. Treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
The US Leader in Stem Cell Therapy, Now in Mexico. Affordable treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
Written by Dr. David Greene, MD, PhD, MBA on July 30, 2025
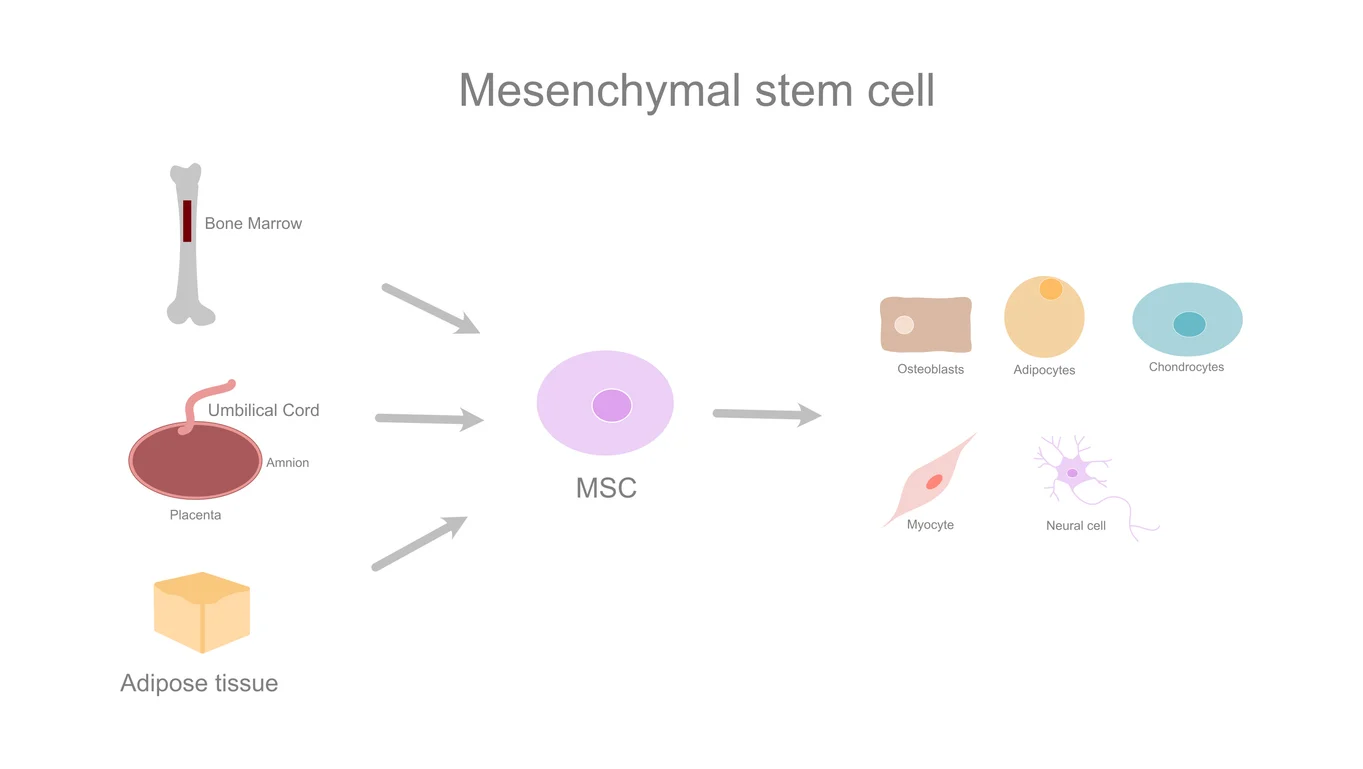
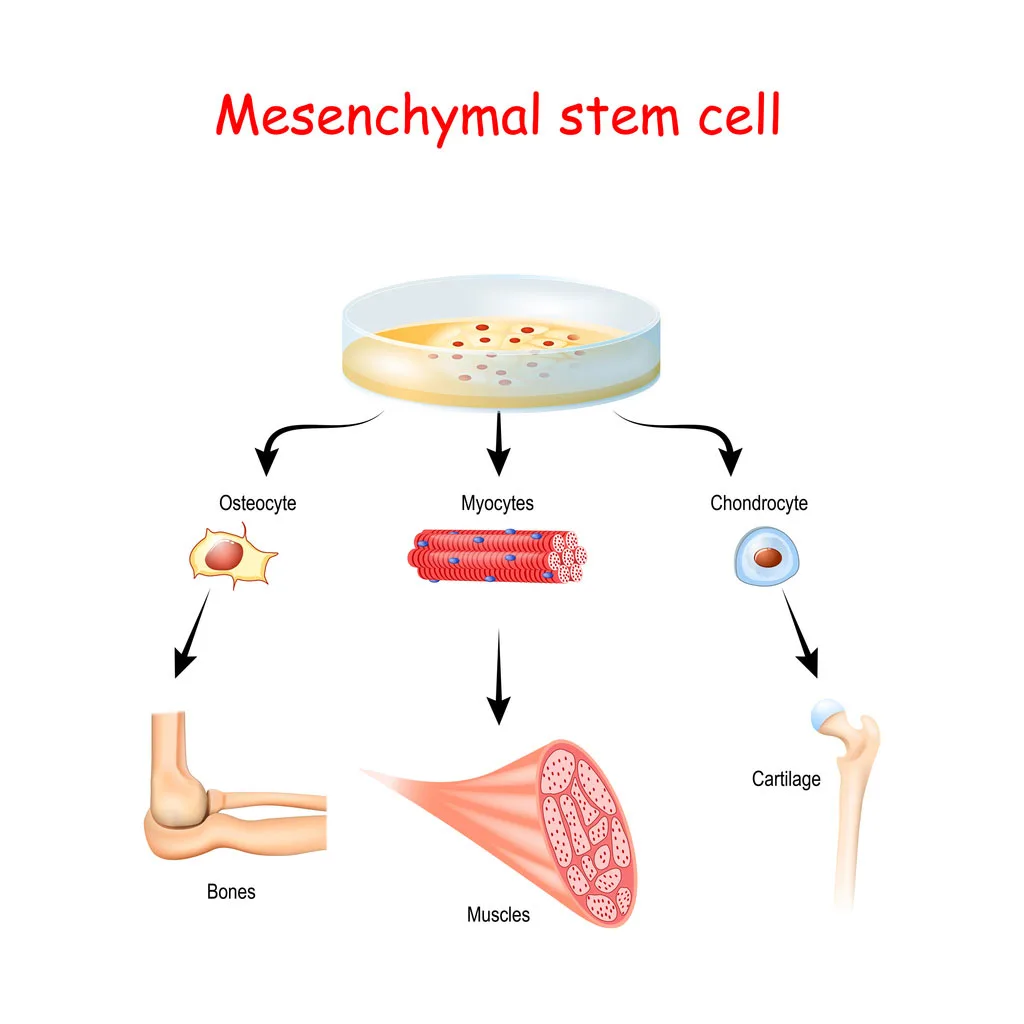
Mesenchymal stem cells (MSCs) are multipotent stromal cells capable of differentiating into various cell types, including osteoblasts (bone), chondrocytes (cartilage), myocytes (muscle), and adipocytes (fat).
The term mesenchymal stem cell was introduced in 1991 by Arnold Caplan.
“Mesenchymal stem cells (MSCs) are adult stem cells with the ability to differentiate into various types of mesoderm-derived cells, showing promising results in preclinical studies for various medical conditions.”
MSCs often represent a heterogeneous population; only a subset shows true multipotency.
Criterion
Description
Plastic adherence
Retention in standard culture
Surface markers (+)
CD105, CD73, CD90 ≥95%+
Surface markers (−)
CD45, CD34, CD14/CD11b, CD79α/CD19, HLA-DR <2%+
Differentiation
Bone, cartilage, and fat differentiation in vitro
Cancer Trials
“Among MSC-based therapies, the use of MSCs as Trojan horses to deliver therapeutic factors represents an important step forward to a more efficient cancer treatment.”
Bone marrow (most established source), adipose (fat) tissue, umbilical cord (Wharton's jelly), cord blood, placenta, menstrual blood, dental pulp, synovial fluid, muscle, hair follicles, and teeth.
Adipose-derived MSCs are safer and produce larger yields than bone marrow.
Wharton's Jelly MSCs offer primitive cells in large numbers, often from normally discarded tissue after childbirth.
In amniotic fluid, up to 1 in 100 cells from amniocentesis are MSCs.
Immunomodulation
MSCs secrete factors (TGFβ, IDO, IL-10, TNFα, IFNγ, PGE2, nitric oxide) that suppress tumor growth, reduce inflammation, and regulate immune responses. This enables their use in autoimmune diseases, GvHD, Crohn’s, MS, lupus, systemic sclerosis, and RA.
Homimg Ability
MSCs use CXCL12/CXCR4 and multiple chemokine receptors (CCR1–10, CXCR1–6) for site-specific migration.
Clinical Results
The EBMT MSC Expansion Consortium (2006) treated severe GvHD in 40 patients with a median MSC dose of 1.0×10^6 cells/kg, achieving 19 complete remissions with no adverse events.
“As of January 2025, [the Pittenger et al. 1999 Science] paper has been cited over 30,000 times.”
Pediatric Bone Disorders
Horwitz et al. (2002): Engraftment post-BMT led to growth acceleration in 5/6 children with osteogenesis imperfecta.
Neurological Disorders
Koc et al. (2002): Four of six children with metachromatic leukodystrophy showed improved nerve conduction after MSC infusion.
Cancer & Regenerative Medicine
MSCs can act as 'Trojan horses' to deliver engineered agents (e.g., IFNβ, interleukins, chemotherapeutics) directly to tumors, increasing effectiveness and reducing systemic toxicity.
Scaffold and hydrogel technologies bolster MSC retention and anti-tumor effects.
Safety
To date, infusion into over a hundred patients—including children—has resulted in no serious adverse events, supporting a strong safety profile.
Anti-Tumor Effects: MSCs induce tumor cell apoptosis via TRAIL, downregulate PI3K/AKT, inhibit ERK1/2, and block Wnt and angiogenic signaling.
Pro-Tumor Risks: MSCs may also promote tumor growth through secretion of CCL5, VEGF, TGFβ, IL-6, and potential differentiation into cancer-associated fibroblasts, which support the tumor microenvironment.
Clinical Context: The effect depends strongly on MSC source, patient characteristics, cancer type, dosing, and delivery route.
“Bone marrow has a limited mesenchymal stem cell pool that depletes with age and after disease. … This is one major reason bones fail to regenerate in osteoporotic conditions.”
Category
Data/Findings
ISCT Definition (2006)
CD105+, CD73+, CD90+, and CD45−, CD34−, CD14/CD11b−, CD79α/CD19−, HLA-DR−
Peer-reviewed MSC papers (2025)
>80,000
Clinical trials listed (May 2024)
>1,760 studies for over 920 conditions
MSCs Clinical Research (Cancer)
≥14 cancer therapy trials (2020); CELYVIR: 1 pediatric full remission (3 years)
MSC Dosing (GvHD trial, 2006)
Median: 1.0×10^6 cells/kg (range: 0.4–9×10^6)
Adipose MSC yield
Higher & easier extraction than bone marrow
Amniotic MSCs
1 in 100 cells in amniotic fluid may be MSCs
Pittenger et al. (1999) Citations (2025)
>30,000
Wikipedia contributors. (2025, July 18). Mesenchymal stem cell. Wikipedia. https://en.wikipedia.org/wiki/Mesenchymal_stem_cell
Narzisi, A., Halladay, A., Masi, G., Novarino, G., & Lord, C. (2023). Tempering expectations: considerations on the current state of stem cells therapy for autism treatment. Frontiers in psychiatry, 14, 1287879. https://doi.org/10.1038/s41536-019-0083-6
Ding, D. C., Shyu, W. C., & Lin, S. Z. (2011). Mesenchymal stem cells. Cell transplantation, 20(1), 5–14. https://doi.org/10.3727/096368910X
Keating A. (2006). Mesenchymal stromal cells. Current opinion in hematology, 13(6), 419–425. https://doi.org/10.1097/01.moh.0000245697.54887.6f
Li, C., Wang, S., Jiang, J., Fu, W., & Zeng, J. (2025). The promise of cell-based therapies in diabetes: A review of mesenchymal stem cell applications and trials. Biochimie, 237, 54–65. https://doi.org/10.1016/j.biochi.2025.07.011
Liu, J., Gao, J., Liang, Z. et al. Mesenchymal stem cells and their microenvironment. Stem Cell Res Ther 13, 429 (2022). https://doi.org/10.1186/s13287-022-02985-y
Zhuang, WZ., Lin, YH., Su, LJ. et al. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci 28, 28 (2021). https://doi.org/10.1186/s12929-021-00725-7
Hmadcha, A., Martin-Montalvo, A., Gauthier, B. R., Soria, B., & Capilla-Gonzalez, V. (2020). Therapeutic potential of mesenchymal stem cells for cancer therapy. Frontiers in Bioengineering and Biotechnology, 8, Article 43. https://doi.org/10.3389/fbioe.2020.00043
Han, Y., Li, X., Zhang, Y., Han, Y., Chang, F., & Ding, J. (2019). Mesenchymal Stem Cells for Regenerative Medicine. Cells, 8(8), 886. https://doi.org/10.3390/cells8080886
Arnold I. Caplan, Mesenchymal Stem Cells: Time to Change the Name!, Stem Cells Translational Medicine, Volume 6, Issue 6, June 2017, Pages 1445–1451, https://doi.org/10.1002/sctm.17-0051
Williams, A. R., Hare, J. M., Dimmeler, S., & Losordo, D. (2011). Mesenchymal stem cells. Circulation Research, 109(8), 923–940. https://doi.org/10.1161/CIRCRESAHA.111.243147
Fitzsimmons, R. E. B., Mazurek, M. S., Soos, A., & Simmons, C. A. (2018). Mesenchymal Stromal/Stem cells in regenerative medicine and tissue engineering. Stem Cells International, 2018, 1–16. https://doi.org/10.1155/2018/8031718
Wang, L.-T., Ting, C.-H., Yen, M.-L., Liu, K.-J., Sytwu, H.-K., Wu, K. K., & Yen, B. (2016). Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. Journal of Biomedical Science, 23, Article 76. https://doi.org/10.1186/s12929-016-0289-5
Contact US

Dr. David Greene
MD, PhD, MBA
Dr. David Greene, MD, PhD, MBA, is a pioneering leader in regenerative medicine and healthcare marketing. As a residency and fellowship-trained orthopedic surgeon, Dr. Greene transitioned from clinical practice to become the founder and CEO of R3 Stem Cell and US Lead Network, where he has revolutionized patient care and medical practice growth through innovative therapies and digital marketing strategies. He has authored two influential books on healthcare internet marketing, ranks among the top expert authors globally, and has been featured on the cover of Corporate Vision magazine for his impact on global regenerative therapies. Beyond his professional achievements, Dr. Greene is passionate about education, compassion, and continuous innovation.

About R3 Stem Cell Mexico
Follow Us
Quick Links
Disclaimer
Stem cell therapy is considered experimental and is regulated by the U.S. Food and Drug Administration (FDA), but it is not FDA-approved. R3 Stem Cell does not offer stem cell therapy as a cure for any medical condition. No statements made on this site have been evaluated or approved by the FDA. This site does not provide medical advice. All content is for informational purposes only and is not a substitute for professional medical consultation, diagnosis, or treatment. Reliance on any information provided by R3 Stem Cell, its employees, others appearing on this website at the invitation of R3 Stem Cell, or other visitors to the website is solely at your own risk. R3 Stem Cell does not recommend or endorse any specific tests, products, procedures, opinions, or other information that may be mentioned on this website. R3 Stem Cell is not responsible for the outcome of your procedure. The FDA considers stem cell therapy experimental at this point.
Contact Us










Copyright © 2016 – 2025 R3 Stem Cell