Affordable & Advanced Tijuana Stem Cell Treatment
At R3 Stem Cell Tijuana, we help patients repair damaged joints, reduce chronic pain, and improve mobility – all without surgery or lengthy recovery times.
- Repair
- Regenerate
- Repair
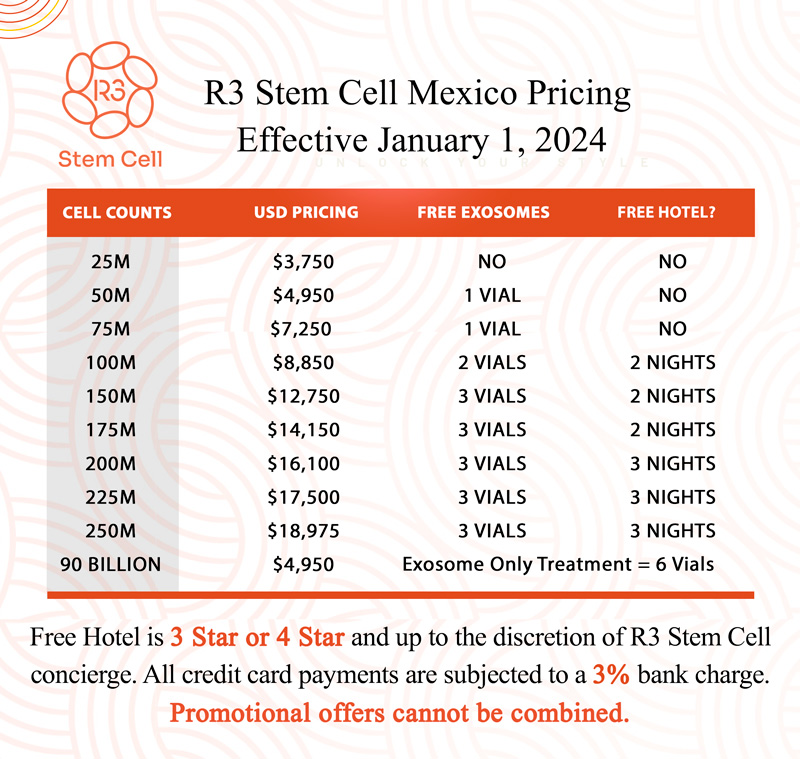
The US Leader in Stem Cell Therapy, Now in Mexico. Treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!
The US Leader in Stem Cell Therapy, Now in Mexico. Affordable treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
At R3 Stem Cell Tijuana, we help patients repair damaged joints, reduce chronic pain, and improve mobility – all without surgery or lengthy recovery times.


Our team handles everything from airport pickup to aftercare instructions. You focus on recovery – we handle the details.
Schedule Your Stem Cell Therapy at Our Tijuana Clinic Now at: 888-988-0515.
Wondering what happens after you call? Here’s how we make stem cell therapy in Tijuana simple:
Free Phone Consultation
Talk to our specialists in 15 minutes. No pressure, just answers.
Custom Plan
Get your Tijuana stem cell therapy customized to YOUR specific needs.
Easy Travel
We handle airport pickup, hotel stays, and clinic rides for optimal convenience.
Our stem cell procedures include:

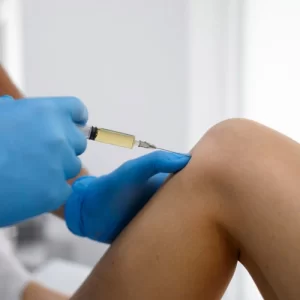
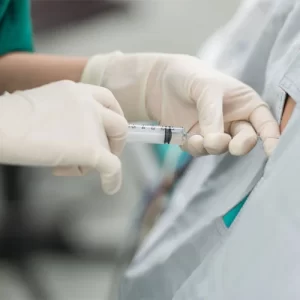
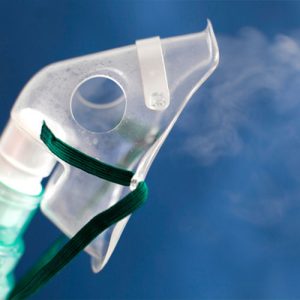
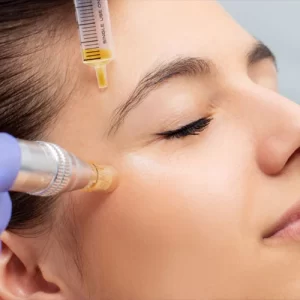
Many patients experience relief from joint pain, improved mobility, reduced inflammation, and better overall well-being. The extent of improvement varies based on the condition, treatment plan, and individual response.

Mexican Health Authority-approved facility
Strict sterility and quality control measures
Experienced doctors with extensive stem cell expertise
Personalized screening to ensure patient safety
Don’t gamble with inexperienced clinics.
R3’s COFEPRIS-accredited facility can turn a “maybe” into “I feel much better” for $0 upfront.
Detail
Tijuana Clinic
Typical US Clinic
$3,750+
$5,000-$50,000+
Up to 250 million live stem cells per treatment
Varies; often lower cell counts
2-7 days
Wait times for stem cell therapy in U.S. clinics vary widely depending on the clinic and treatment type

Faster Recovery
Faster Recovery
Precision Targeting
Precision Targeting
Data-Backed Approach
Data-Backed Approach
Many patients report noticeable improvements in mobility, pain levels, and overall well-being after treatment. Results vary based on individual health conditions, treatment plans, and response to therapy.
Find out if you’re a candidate for stem cell Treatment in Tijuana, Mexico—dial 888-988-0515 today!
Yes. Clinics such as R3 Stem Cell Tijuana have performed over 25,000 procedures in the past decade, with a patient satisfaction rate exceeding 85% and no reported major complications. However, avoid unregulated clinics—recent CDC warnings highlight risks of drug-resistant infections from poorly administered treatments. Always verify clinic credentials and safety protocols.
Costs start at $3,750 for 25 million cells at R3, up to $18,975 for higher cell counts. Our Tijuana clinic offers high-quality treatments at a fraction of the cost compared to clinics in the U.S., with savings of up to 50% or more depending on the procedure.
R3 Stem Cell Tijuana: COFEPRIS-certified, High cell counts (200 million+), accredited facilities, and 85% satisfaction rates.
Yes, via COFEPRIS (Mexico’s health authority). Reputable clinics meet GMP standards and FDA-equivalent guidelines. However, enforcement varies—some clinics operate without proper oversight.
Common conditions include:
Yes. Top clinics employ board-certified specialists trained in the U.S. and Mexico.
Most patients resume light activity same-day. Results may take 2-3 months as cells integrate.
Minor risks include temporary swelling or soreness. Severe risks (e.g., infections) are rare at accredited clinics but have occurred at unregulated facilities. Always ask about infection rates and cell sourcing.
For many, yes. Savings of $10,000+ compared to many U.S. prices, combined with high-quality care at top clinics, make Tijuana a cost-effective option. Ensure clinics provide transparent pricing and post-treatment support.
It uses cells to repair damaged tissues. Types include umbilical cord and adipose-derived cells, injected or infused to reduce inflammation and promote healing.
At R3 Stem Cell Tijuana, we carefully screen patients to ensure safety and effectiveness. Ideal candidates include those with joint pain, autoimmune disorders, neurological conditions, and metabolic diseases. Not all patients qualify for treatment, as eligibility is determined based on medical history, condition severity, and overall health to ensure safety and effectiveness.
The effectiveness of stem cell therapy depends on the condition being treated and individual factors. Studies have shown promising outcomes, particularly for joint issues, autoimmune conditions, and neurological disorders. Many patients report noticeable improvements in pain relief, function, and overall quality of life.
Cells and exosomes are administered in many different ways depending on the condition. Some examples may include: injections (e.g., knees), intravenous infusions, intrathecal placements, nebulizer techniques or intranasal applications. R3 uses ultrasound-guided technique for precision and accuracy.
At R3 Stem Cell Tijuana, we prioritize ongoing support. Our team follows up with patients at 1, 3, and 6 months to track progress and address any concerns. While most patients require little downtime, we provide guidance to optimize recovery and maximize results.
R3 Stem Cell Tijuana
Hours
References