Hematopoietic Stem Cell Transplantation: A Comprehensive Medical Analysis
Written by Dr. David Greene, MD, PhD, MBA on July 28, 2025
The US Leader in Stem Cell Therapy, Now in Mexico. Treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
The US Leader in Stem Cell Therapy, Now in Mexico. Affordable treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
Written by Dr. David Greene, MD, PhD, MBA on July 28, 2025
Hematopoietic stem cell transplantation (HSCT) is a life-saving treatment used to replace damaged or diseased blood-forming cells with healthy stem cells. It’s commonly used for conditions like blood cancers (e.g., leukemia and lymphoma), immune system disorders, and inherited blood diseases such as sickle cell anemia or thalassemia.
Each year, doctors perform over 25,000 HSCT procedures worldwide, helping patients recover by restoring healthy blood cell production. The stem cells used in these transplants are either collected from the patient’s own body (autologous transplant) or from a matched donor (allogeneic transplant). These powerful cells can regenerate into red blood cells, white blood cells, and platelets, rebuilding the immune system and improving health outcomes.
Research from top institutions like the Fred Hutchinson Cancer Center shows that success rates are higher when transplants are done at specialized, high-volume centers with experienced teams.
Since the first transplant in 1957, more than 1.5 million HSCTs have been completed worldwide. In fact, the global medical community celebrated the one millionth transplant milestone back in 2012, according to the Worldwide Network for Blood and Marrow Transplantation.
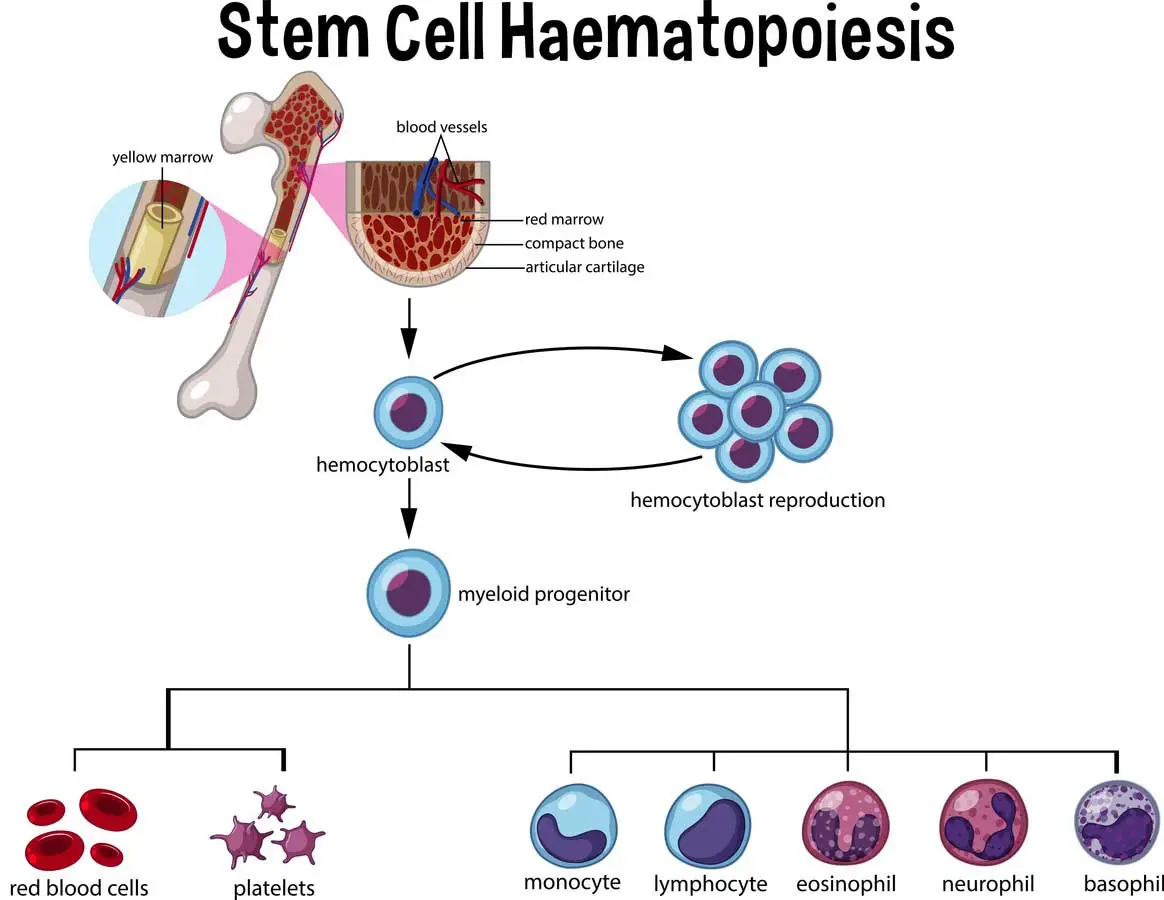
The bone marrow produces all blood cells through hematopoietic stem cells. These master cells divide and mature into different blood cell types that your body needs to function properly. When disease damages this system, stem cell transplantation can restore normal blood production.
Stem cells are collected from three main sources: peripheral blood, bone marrow, and umbilical cord blood. Peripheral blood stem cells have become the most common source, accounting for 77-80% of all procedures. The cells must be carefully processed and stored to maintain their life-saving potential.
Prior to the transplant, patients receive high-dose chemotherapy and radiation therapy. This conditioning destroys diseased cells and creates space for new stem cells to grow. The graft-versus-tumor effect occurs when donor cells actively fight remaining cancer cells in the recipient’s body.
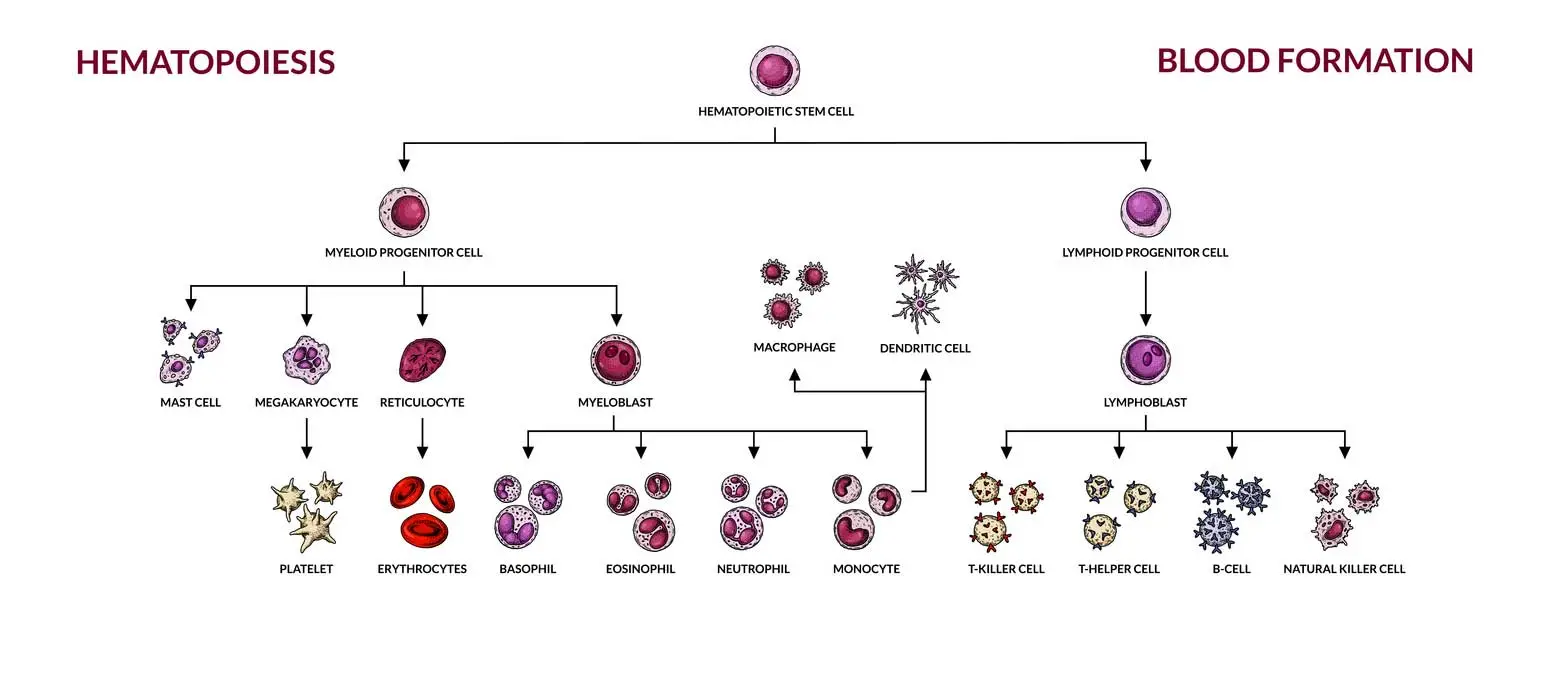
Autologous transplants use the patient’s own stem cells collected before treatment begins. This approach eliminates the risk of graft-versus-host disease since no foreign cells enter the body. However, autologous procedures carry a higher risk of cancer relapse compared to donor transplants.
The patient’s own stem cells are harvested through a process called apheresis. Medical teams inject growth factors to increase stem cell numbers in the blood. Peripheral blood stem cell collection typically takes 4-5 hours over several days.
In the case of autologous transplants, patients with lymphoma (relapsed, refractory) show cure rates reaching 50-60%. Multiple myeloma patients frequently receive this treatment, with nearly 8,000 procedures performed annually in the U.S. alone.
Allogeneic transplants involve stem cells from a related or unrelated donor. The donor and recipient must share compatible tissue types to reduce rejection risks. Finding a suitable donor requires extensive genetic testing through donor registries.
Less than 30% of patients have an HLA-identical sibling donor available. The remaining patients must search through registries containing over 20.2 million registered donors worldwide. Cord blood banks provide additional options, especially for pediatric patients.
R3 Stem Cell Mexico maintains rigorous standards for donor matching and cell processing, following international protocols established by leading transplant organizations. The facility uses advanced HLA typing technology to identify the most compatible donors for each patient.
Allogeneic transplants involve stem cells from a related or unrelated donor. The donor and recipient must share compatible tissue types to reduce rejection risks. Finding a suitable donor requires extensive genetic testing through donor registries.
Less than 30% of patients have an HLA-identical sibling donor available. The remaining patients must search through registries containing over 20.2 million registered donors worldwide. Cord blood banks provide additional options, especially for pediatric patients.
R3 Stem Cell Mexico maintains rigorous standards for donor matching and cell processing, following international protocols established by leading transplant organizations. The facility uses advanced HLA typing technology to identify the most compatible donors for each patient.
The spectrum of HSCT procedures varies significantly across different regions. North America leads with 560.8 transplants per 10 million population, followed by Europe at 438.5 per 10 million. Latin America shows growing numbers with 76.7 procedures per 10 million residents.
Recent data shows that 82,718 first-time HSCTs were reported worldwide in 2016, representing a 6.2% increase in autologous procedures and 7.0% growth in allogeneic transplants. The majority (53.5%) were autologous transplants.
Haploidentical transplant rates were higher in Southeast Asia and Western Pacific regions (32%) compared to North America (4.9%). This variation reflects different medical practices and donor availability across continents.
HSCT treats a wide range of blood cancers and immune system disorders. Acute and chronic leukemia represent major indications, along with multiple myeloma and various lymphomas. The procedure also helps patients with non-malignant conditions such as severe aplastic anemia and sickle cell disease.
Available data from 2016-2020 suggests approximately 1,200 allogeneic AML transplants are performed annually in the U.S. These procedures can offer cure or long-term remission for some patients who would otherwise have limited treatment options.
Research indicates that HSCT may provide curative treatment for some chemotherapy-resistant blood cancers, though outcomes vary significantly by cancer type and patient factors. Some patients who would otherwise face terminal diagnoses can achieve remission through this procedure, though success rates for chemoresistant cases remain modest.
HSCT treats a wide range of blood cancers and immune system disorders. Acute and chronic leukemia represent major indications, along with multiple myeloma and various lymphomas. The procedure also helps patients with non-malignant conditions such as severe aplastic anemia and sickle cell disease.
Available data from 2016-2020 suggests approximately 1,200 allogeneic AML transplants are performed annually in the U.S. These procedures can offer cure or long-term remission for some patients who would otherwise have limited treatment options.
Research indicates that HSCT may provide curative treatment for some chemotherapy-resistant blood cancers, though outcomes vary significantly by cancer type and patient factors. Some patients who would otherwise face terminal diagnoses can achieve remission through this procedure, though success rates for chemoresistant cases remain modest.
Survival rates for stem cell transplantation vary significantly based on patient age, disease type, and donor compatibility. Recent data shows 100-day post-HSCT survival reaches 78% across all patient groups, while 1-year survival maintains 66% and stabilizes at the same rate for 3-year outcomes.
Early mortality within 100 days affects approximately 4% of patients for both autologous and allogeneic procedures. However, late complications and non-relapse mortality rates can reach as high as 46% in some patient populations. These statistics highlight the importance of careful patient selection and experienced medical teams.

For severe aplastic anemia in children, 5-year survival rates approach 90% with unrelated donors and reach 100% with matched sibling transplants. Event-free survival at 2 years shows clear risk stratification: low-risk patients achieve 86% survival, intermediate-risk reach 71%, and high-risk patients maintain 54% based on comorbidity assessments.
The risk of cancer relapse remains a primary concern throughout the recovery period. Autologous transplants show higher relapse rates but lower treatment-related mortality compared to allogeneic procedures. Studies indicate that the graft versus tumor effect in allogeneic transplants reduces relapse risk by 30-40% through active immune surveillance.
For severe aplastic anemia in children, 5-year survival rates approach 90% with unrelated donors and reach 100% with matched sibling transplants. Event-free survival at 2 years shows clear risk stratification: low-risk patients achieve 86% survival, intermediate-risk reach 71%, and high-risk patients maintain 54% based on comorbidity assessments.
The risk of cancer relapse remains a primary concern throughout the recovery period. Autologous transplants show higher relapse rates but lower treatment-related mortality compared to allogeneic procedures. Studies indicate that the graft versus tumor effect in allogeneic transplants reduces relapse risk by 30-40% through active immune surveillance.
Graft-versus-host disease represents the most serious complication of allogeneic transplants, affecting 40-60% of recipients. Acute GVHD develops within 100 days and primarily targets skin, liver, and digestive tract. The mucosal lining of the intestines becomes inflamed, causing severe diarrhea and nutrition problems.
The recipient’s immune system receives completely new cells that may recognize the patient’s tissues as foreign. This reaction creates a complex balance between beneficial graft-versus-tumor effects and harmful tissue damage. Chronic GVHD may develop months or years later, affecting 50% of long-term survivors after HLA-identical sibling transplant procedures.
Treatment of graft-versus-host disease requires careful management with immunosuppressive medications. Doctors must balance controlling GVHD symptoms while maintaining the cancer-fighting benefits of donor cells. Doses of immunosuppressive agents require frequent adjustment based on patient response and side effect profiles.
Severe cases of acute GVHD (grade III-IV) carry poor prognosis, with 2-year survival rates of 25-30% for grade III and only 1-2% for grade IV disease. These statistics underscore the importance of prevention strategies and early intervention when symptoms develop.
Prior to the actual cell infusion, patients undergo intensive conditioning regimens designed to eliminate diseased cells and suppress the immune system. Chemotherapy and radiation therapy destroy existing bone marrow cells to create space for new stem cells to engraft successfully.
Total body irradiation delivers precisely calculated radiation doses to the entire body, targeting cancer cells while preparing the bone marrow environment. The conditioning phase typically lasts 7-10 days and requires hospitalization for close monitoring and supportive care.
Reduced-intensity conditioning regimens allow older patients or those with organ dysfunction to receive transplants safely. These modified approaches reduce immediate toxicity but may increase the risk of cancer relapse compared to full-intensity treatments.
Peripheral blood stem cell collection has become the standard approach for most transplant procedures. Donors receive G-CSF injections for 4-5 days to mobilize stem cells from the bone marrow into the bloodstream. As a result of G-CSF stimulation, stem cell numbers increase dramatically, allowing efficient collection.
The collection process, called apheresis, separates stem cells from other blood components over several hours. The cells must be processed immediately to maintain viability and function. Advanced processing techniques ensure optimal cell counts and reduce contamination risks.
Bone marrow and cord blood represent alternative stem cell sources for specific patient populations. Bone marrow harvest yields approximately 2.5 × 10^8 nucleated cells per kilogram of recipient weight, while cord blood units typically contain lower cell doses but offer reduced GVHD risks.
The actual stem cell infusion resembles a blood transfusion and typically takes 1-4 hours depending on cell volume. Patients receive the cells through a central venous catheter while medical teams monitor for immediate reactions. The procedure itself is generally well-tolerated with minimal discomfort.
Stem cells from a donor travel naturally through the bloodstream to bone marrow spaces where they begin establishing new blood cell production. This homing process relies on chemical signals that guide cells to appropriate locations within the bone marrow environment.
Engraftment monitoring begins immediately after infusion through daily blood counts and bone marrow assessments. White blood cells typically recover first within 2-3 weeks, followed by platelet recovery in 3-4 weeks. Complete immune system restoration may require 6-12 months or longer.
The actual stem cell infusion resembles a blood transfusion and typically takes 1-4 hours depending on cell volume. Patients receive the cells through a central venous catheter while medical teams monitor for immediate reactions. The procedure itself is generally well-tolerated with minimal discomfort.
Stem cells from a donor travel naturally through the bloodstream to bone marrow spaces where they begin establishing new blood cell production. This homing process relies on chemical signals that guide cells to appropriate locations within the bone marrow environment.
Engraftment monitoring begins immediately after infusion through daily blood counts and bone marrow assessments. White blood cells typically recover first within 2-3 weeks, followed by platelet recovery in 3-4 weeks. Complete immune system restoration may require 6-12 months or longer.
The immune system remains severely compromised for months following stem cell transplantation, creating significant infection risks. Bacterial infections occur in up to 30% of patients post-transplant, while viral reactivations affect 26.2% of allogeneic recipients compared to 2.9% of autologous patients.
CMV reactivation occurs in approximately 30% of allogeneic recipients, carrying a mortality rate of 46% for affected patients. Invasive aspergillosis develops in roughly 5% of autologous transplant patients and up to 30% of allogeneic recipients, requiring aggressive antifungal therapy.
For the prevention of serious infections, patients receive prophylactic antibiotics, antiviral medications, and antifungal drugs throughout the vulnerable period. Isolation precautions include positive-pressure rooms and strict visitor restrictions during the immediate post-transplant phase.
R3 Stem Cell Mexico implements comprehensive infection control protocols based on international guidelines. The facility maintains specialized isolation units with advanced air filtration systems and rigorous staff training in infection prevention techniques.
High-dose conditioning regimens can cause organ damage affecting heart, lung, liver, and kidney function. Pulmonary complications represent the most life-threatening issues, with 15-40% of HSCT recipients potentially requiring intensive care unit admission during recovery.
The development of secondary cancers represents a long-term risk following stem cell transplantation. Radiation and chemotherapy exposure during conditioning increases cancer risk, particularly skin cancers and solid tumors that may develop years after treatment.
Fertility preservation requires discussion prior to treatment since conditioning regimens frequently cause permanent reproductive dysfunction. Young patients often undergo sperm banking or egg harvesting before beginning the transplant process to preserve future family planning options.
CMV reactivation occurs in approximately 30% of allogeneic recipients, carrying a mortality rate of 46% for affected patients. Invasive aspergillosis develops in roughly 5% of autologous transplant patients and up to 30% of allogeneic recipients, requiring aggressive antifungal therapy.
For the prevention of serious infections, patients receive prophylactic antibiotics, antiviral medications, and antifungal drugs throughout the vulnerable period. Isolation precautions include positive-pressure rooms and strict visitor restrictions during the immediate post-transplant phase.
R3 Stem Cell Mexico implements comprehensive infection control protocols based on international guidelines. The facility maintains specialized isolation units with advanced air filtration systems and rigorous staff training in infection prevention techniques.
Hospital stays average 14 days for autologous transplants and 30 days for allogeneic procedures, though complications can extend these periods significantly. Most patients achieve initial engraftment within 30 days, marking the first major recovery milestone.
Serious adverse events requiring prolonged hospitalization occur in approximately 0.6% of stem cell donors, while serious cardiovascular reactions affect about 0.07% of cases. These low rates reflect improved safety protocols and donor screening procedures.
The worldwide network for blood and marrow transplantation maintains extensive registries tracking long-term outcomes and complications. Data collection helps identify risk factors and improve treatment protocols for future patients requiring stem cell transplantation.
Hospital stays average 14 days for autologous transplants and 30 days for allogeneic procedures, though complications can extend these periods significantly. Most patients achieve initial engraftment within 30 days, marking the first major recovery milestone.
Serious adverse events requiring prolonged hospitalization occur in approximately 0.6% of stem cell donors, while serious cardiovascular reactions affect about 0.07% of cases. These low rates reflect improved safety protocols and donor screening procedures.
The worldwide network for blood and marrow transplantation maintains extensive registries tracking long-term outcomes and complications. Data collection helps identify risk factors and improve treatment protocols for future patients requiring stem cell transplantation.

Long-term survivors of stem cell transplantation face unique challenges that extend far beyond the initial recovery period. Studies indicate that 80.8% of patients experience some degree of complications during their treatment journey, with allogeneic HSCT showing higher complication rates (88.9%) compared to autologous procedures (56.3%).
The recipient’s immune system requires extensive time to rebuild normal function, often taking 12-24 months to achieve adequate protection against common infections. During this vulnerable period, patients must limit exposure to crowds, avoid certain foods, and maintain strict hygiene practices that significantly impact daily activities.
Chronic fatigue affects the majority of long-term survivors, with many patients reporting persistent tiredness months or years after successful engraftment. This condition often improves gradually but may require ongoing medical management and lifestyle adjustments to maintain acceptable activity levels.
Bone pain from G-CSF mobilization affects 84% of donors during the collection process, while headache (54%) and fatigue (31%) represent additional common symptoms. These temporary effects typically resolve within days of completing the donation process.
Psychological distress commonly develops during the extended treatment period, affecting both patients and family members. The uncertainty surrounding outcomes, combined with prolonged isolation and physical discomfort, creates significant emotional challenges requiring professional support services.
Many transplant centers now provide comprehensive survivorship programs addressing the unique needs of HSCT recipients. These programs coordinate medical follow-up, monitor for late complications, and provide resources for managing the long-term effects of treatment.
The financial impact of stem cell transplantation extends far beyond the immediate procedure costs. Median total healthcare expenses at 100 days post-HSCT range from $140,000 for autologous procedures to $290,000 for allogeneic transplants with myeloablative conditioning, according to 2017 estimates.
Pediatric HSCT typically costs more than adult procedures due to longer hospital stays, increased complication rates, and specialized care requirements. Insurance coverage varies significantly, with some plans requiring extensive pre-authorization processes that can delay critical treatment.
Hidden costs include lost income during treatment and recovery, travel expenses for families seeking specialized care, and ongoing medical monitoring that continues for years after transplant. Many families face financial hardship despite insurance coverage due to these additional expenses.
R3 Stem Cell Mexico provides high-quality HSCT services at significantly reduced costs compared to many developed countries. The facility maintains international accreditation standards while offering treatment packages that include comprehensive care coordination and support services.
Medical tourism for stem cell transplantation has grown substantially, with patients seeking affordable options without compromising safety or effectiveness. Careful evaluation of international programs ensures patients receive appropriate care while managing costs effectively.
Treatment abroad requires careful planning for extended stays, language barriers, and coordination with home healthcare providers for ongoing follow-up care. Success depends heavily on selecting experienced facilities with proven track records in HSCT procedures.
Chimeric antigen receptor T-cell therapy represents a revolutionary approach that modifies the patient’s own immune cells to fight cancer more effectively. This technology complements traditional stem cell transplantation by providing additional treatment options for resistant blood cancers.
Clinical trials increasingly combine CAR T-cell therapies with reduced-intensity conditioning regimens, potentially reducing transplant-related toxicity while maintaining therapeutic effectiveness. Early results suggest these combination approaches may improve outcomes for high-risk patients.
The development of off-the-shelf CAR T-cell products could eliminate the need for individualized cell manufacturing, reducing treatment delays and costs while expanding access to this promising therapy.
High-resolution HLA typing now examines 10-12 genetic markers compared to earlier methods that evaluated fewer compatibility factors. This precision matching reduces acute graft-versus-host disease rates from 45% to less than 25% in unrelated donor transplants.
Artificial intelligence algorithms help identify optimal donor matches from global registries containing millions of potential candidates. These systems analyze complex genetic data to predict compatibility and transplant outcomes more accurately than traditional matching methods.
Haploidentical transplantation techniques allow the use of half-matched family donors, significantly expanding the pool of available donors. Post-transplant cyclophosphamide protocols have made these procedures safer and more effective, particularly benefiting patients from diverse ethnic backgrounds.
Advanced cell processing techniques improve the quality and safety of transplanted stem cells. Automated systems reduce contamination risks while standardizing processing procedures across different transplant centers worldwide.
Ex vivo expansion methods multiply stem cell numbers before transplantation, potentially reducing the minimum cell dose required for successful engraftment. These techniques may allow the use of smaller cord blood units and improve outcomes in larger adult recipients.
Gene editing technologies show promise for correcting genetic defects in stem cells before transplantation. Early clinical trials target inherited immune deficiencies and blood disorders, potentially providing curative treatments without requiring donor cells.
New antimicrobial agents specifically designed for immunocompromised patients show promise in preventing serious post-transplant infections. These medications target resistant organisms while minimizing toxicity to recovering immune systems.
Microbiome research reveals the importance of intestinal bacteria in transplant outcomes. Fecal microbiota transplantation and probiotic therapies may reduce infection risks and improve overall recovery in HSCT recipients.
Rapid diagnostic testing allows earlier detection of viral reactivations and opportunistic infections. Point-of-care testing devices provide results within hours rather than days, enabling prompt treatment initiation when infections develop.

Targeted conditioning regimens deliver cytotoxic therapy directly to bone marrow while sparing other organs from damage. Radioimmunotherapy conjugates use antibodies to guide radiation specifically to blood-forming tissues, reducing overall toxicity.
Cellular therapy approaches replace high-dose chemotherapy with immune-based treatments that selectively eliminate diseased cells. These methods may achieve similar anti-cancer effects with substantially less organ damage and improved patient tolerance.
Supportive care medications continue improving, with new drugs preventing mucositis, reducing nausea, and managing other treatment-related side effects. These advances significantly improve patient comfort and quality of life during treatment.
Despite significant advances in stem cell transplantation technology, access remains limited in many parts of the world. Regional disparities in transplant rates reflect differences in healthcare infrastructure, economic resources, and trained medical personnel.
Africa and Eastern Mediterranean regions show transplant rates of only 27.8 per 10 million population compared to 560.8 per 10 million in North America. These gaps highlight the need for expanded training programs and technology transfer to underserved regions.
Donor registry diversity remains a critical challenge, with people of European ancestry representing the majority of registered donors. Minority populations face significantly longer search times and lower match probabilities when seeking unrelated donors for transplantation.
International collaboration programs train medical teams from developing countries in HSCT techniques and establish sustainable transplant programs. These partnerships transfer knowledge while building local capacity for providing life-saving treatments.
Telemedicine platforms connect patients in remote areas with transplant specialists, enabling expert consultation and follow-up care without requiring extensive travel. These technologies democratize access to specialized knowledge and improve outcomes in underserved populations.
Stem cell transplantation continues evolving from an experimental procedure to a standard treatment option for many blood disorders. Over one million procedures have been performed globally, with success rates steadily improving through advances in donor matching, supportive care, and complication management.
The worldwide network for blood and marrow transplantation facilitates global collaboration in research and clinical care, accelerating the development of new treatment approaches. Data sharing between centers enables rapid identification of best practices and improved patient outcomes.
As our understanding of stem cell biology and immune system function advances, new therapeutic approaches will likely emerge. These developments promise to make stem cell transplantation safer, more effective, and accessible to broader patient populations worldwide.
The integration of precision medicine approaches, artificial intelligence, and advanced cell therapies represents the next frontier in HSCT development. These innovations offer hope for patients facing blood cancers and immune disorders while continuing to push the boundaries of what medical science can achieve.
Khaddour, K., Hana, C. K., & Mewawalla, P. (2023, May 6). Hematopoietic stem cell transplantation. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK536951/
Wikipedia contributors. (2025a, July 17). Hematopoietic stem cell transplantation. Wikipedia. https://en.wikipedia.org/wiki/Hematopoietic_stem_cell_transplantation
Hatzimichael, E., & Tuthill, M. (2010). Hematopoietic stem cell transplantation. Stem cells and cloning : advances and applications, 3, 105–117. https://doi.org/10.2147/SCCAA.S6815
Keating A. (2006). Mesenchymal stromal cells. Current opinion in hematology, 13(6), 419–425. https://doi.org/10.1097/01.moh.0000245697.54887.6f
Aljagthmi, A.A., Abdel-Aziz, A.K. Hematopoietic stem cells: Understanding the mechanisms to unleash the therapeutic potential of hematopoietic stem cell transplantation. Stem Cell Res Ther 16, 60 (2025). https://doi.org/10.1186/s13287-024-04126-z
Pandey, T., Thomas, S., & Heller, M. T. (2016). Current indications, techniques, and imaging findings of stem cell treatment and bone marrow transplant. Radiologic Clinics of North America, 54(2), 375–396. https://doi.org/10.1016/j.rcl.2015.09.015
Milone, G., Leotta, S., & Cupri, A. (2025). Hematopoietic Stem Cell Transplantation: Recent Advances. Journal of Clinical Medicine, 14(10), 3379. https://doi.org/10.3390/jcm14103379
Hematopoietic Stem Cell Transplantation and Long-Term Outcomes | Nature Research Intelligence. (n.d.). https://www.nature.com/research-intelligence/nri-topic-summaries/hematopoietic-stem-cell-transplantation-and-long-term-outcomes-micro-18098
Risk Factors for Hematopoietic Stem Cell Transplantation-Associated Bone Loss Khan, Zehva et al. Transplantation and Cellular Therapy, Official Publication of the American Society for Transplantation and Cellular Therapy, Volume 27, Issue 3, 212 - 221
Contact US

Dr. David Greene
MD, PhD, MBA
Dr. David Greene, MD, PhD, MBA, is a pioneering leader in regenerative medicine and healthcare marketing. As a residency and fellowship-trained orthopedic surgeon, Dr. Greene transitioned from clinical practice to become the founder and CEO of R3 Stem Cell and US Lead Network, where he has revolutionized patient care and medical practice growth through innovative therapies and digital marketing strategies. He has authored two influential books on healthcare internet marketing, ranks among the top expert authors globally, and has been featured on the cover of Corporate Vision magazine for his impact on global regenerative therapies. Beyond his professional achievements, Dr. Greene is passionate about education, compassion, and continuous innovation.