What is Peripheral Blood Stem Cell Transplantation
Written by Dr. David Greene, MD, PhD, MBA on July 30, 2025
The US Leader in Stem Cell Therapy, Now in Mexico. Treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
The US Leader in Stem Cell Therapy, Now in Mexico. Affordable treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
Written by Dr. David Greene, MD, PhD, MBA on July 30, 2025
Peripheral blood stem cell transplantation (PBSCT) is a treatment that restores healthy blood-forming stem cells after cancer therapy. To prepare, doctors give growth factor injections like Filgrastim, which move stem cells from the bone marrow into the bloodstream. These cells are then collected through apheresis, a procedure similar to a long blood donation. Once chemotherapy or radiation removes the damaged cells, the stored stem cells are infused back into the body through a simple IV. Inside the bone marrow, they begin producing new red blood cells, white blood cells, and platelets, helping the body recover.
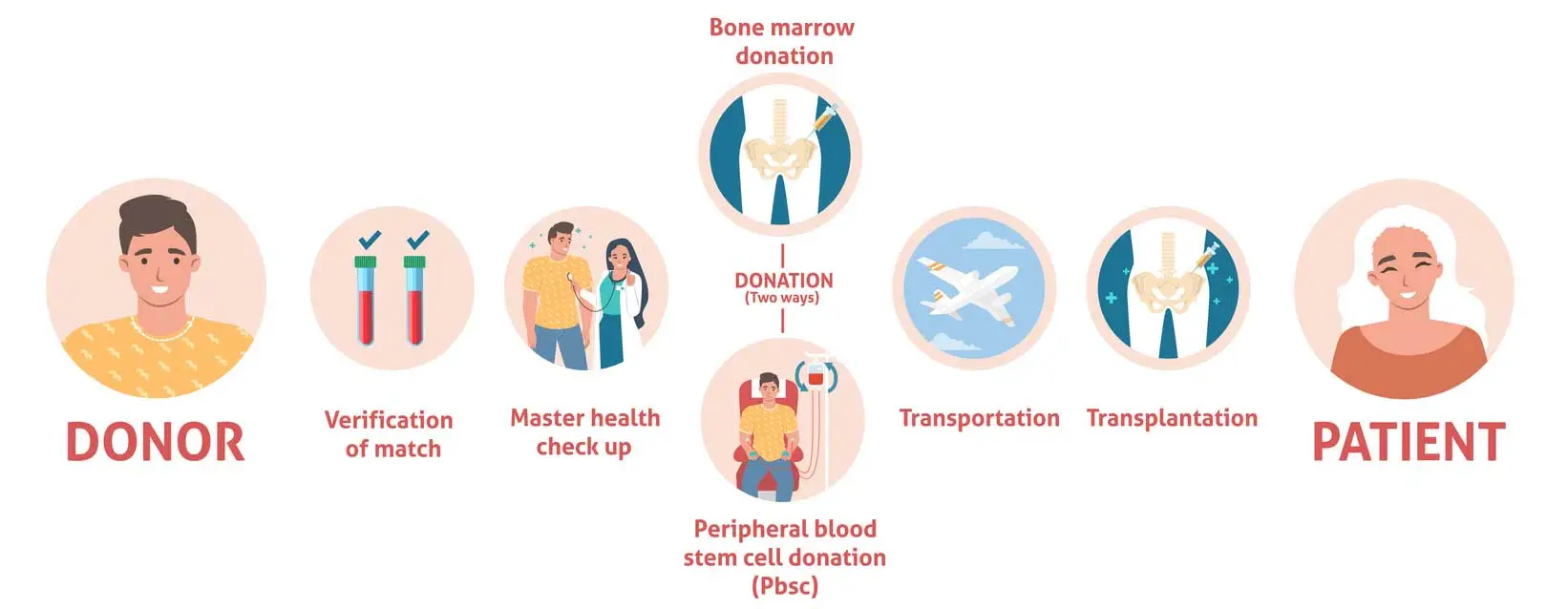
Patients typically must:
Type
Source
Pros
Cons
Syngeneic
Identical twin
No rejection, no GVHD
Rare donor
Haploidentical
Half-matched
Widely available
Requires advanced GVHD control
Region / Year
Total Transplants
Blood-cell Graft Share
1998 worldwide
–
26% of allogeneic grafts
2022 United States
22 576 HCT
≈ 75% of unrelated donor cases
2022–23 China
39 918 HSCT
54% sibling, 77% haplo
The steady climb from one quarter of grafts in 1998 to three quarters in recent American data shows clear trust in the method. R3 Stem Cell Mexico follows these same global standards and tracks every case for quality.
Disease
PBSCT 5-yr OS
Marrow 5-yr OS
Non-Hodgkin Lymphoma
52.7%
56.6%
Hodgkin Disease
52.7%
65.3%
Reduced-intensity haplo-AML (≥60 y)
42%
38%
Study of 1488 donations from 1994-98 recorded only 15 events linked to donation (1%) and zero deaths.
National Marrow Donor Program lists a 0.6 percent rate of serious events during collection.
Such figures confirm the statement by Körbling and Anderlini that PBSCT “is the MOST common transplantation procedure in medicine”. At R3 Stem Cell Mexico every donor receives clear instructions, rapid lab checks, and round-the-clock support.
National Marrow Donor Program lists a 0.6 percent rate of serious events during collection.
Fast engraftment shortens hospital stay and lowers infection risk. This benefit is a key reason many oncologists now pick PBSCT first.
Outcome area
Blood graft (PBSCT)
Bone-marrow graft
Neutrophil recovery (median)
14 days
19 days
Serious donor events
0.6 percent
≤0.5%
Chronic GVHD in aplastic anemia
Relative risk 1.80
Reference
Relapse in reduced-intensity AML age ≥60
Hazard ratio 0.65 (lower)
Reference
Numbers drawn from peer-reviewed trials and registries 1997-2024
R3 Stem Cell Mexico weighs these factors with each patient and explains the plan in plain language.
To give you a feel for how MSCs are used today, here’s where therapy is making waves:
Mobilize and collect
Growth factor shots move stem cells into the blood. Apheresis collects the cells while most blood returns to the donor. One or two sessions fit 85 percent of cases.
Condition
High-dose chemotherapy or radiation clears diseased marrow.
Infuse
Saved cells pass through a standard IV over four to six hours. Nurses watch heart rate and oxygen during the drip.
Engraft
Doctors track CD34+ count and daily blood tests until white cells hit at least 500 per microlitre.
Recover
Average hospital stay ranges from two to three weeks. Patients start daily walks and light meals once counts rise.
Symptom
Why it happens
Typical fix
Bone pain
G-CSF stimulates marrow
Short course of paracetamol or ibuprofen
Nausea / vomiting
Conditioning drugs
Antiemetic pills before meals
Mouth sores
Drop in white cells
Saline rinses, soft diet
Fatigue
Low red cells
Pack-cell transfusion if needed
R3 Stem Cell Mexico keeps a 24-hour hotline for any new fever, rash, or dizziness.
Survival now equals that of marrow grafts in most cancers, yet quality of life hinges on timely GVHD control.
The 1999 Tokaimura criticality accident prompted doctors to give technician Hisashi Ouchi an experimental peripheral-blood stem-cell transplant from his sibling in an effort to restore his devastated marrow and immune system. The procedure temporarily raised his white-cell count, but it did not prevent progressive multiple-organ failure, and Ouchi died 83 days after the exposure. Although the attempt showed that peripheral stem-cell infusion could engraft in a patient with near-total marrow loss, it did not demonstrate proven clinical benefit in such extreme radiation injury cases.
Bring these points to your first consult at stemcellmexico.com.
Main cost parts
Main cost parts
Who pays what
Payer
What they may cover
Key notes
Public insurance (US Medicare, IMSS, others)
Most direct medical costs once the plan approves the transplant
Prior approval forms need lab proof of disease stage
Private insurance
Wide spread in coverage, check yearly cap
Many plans ask for an in-network center
Self pay
Full bill plus travel
Some centers offer package rates and payment plans
Save on hidden items
Before admission
During hospital days
Life in the First Year
Time point
Common task
Goal
Week 1-3
Daily blood draws
Track engraftment
Month 1-3
Taper steroids if GVHD is stable
Cut infection risk
Month 3-6
Restart childhood shots
Rebuild immunity
Month 6-12
Return to part-time work or school
Restore routine
Regular light exercise, such as slow walks, helps fight fatigue. A diet rich in protein and iron speeds red cell growth. Avoid raw fish, unwashed fruit, and crowded events until your doctor clears them.
Wikipedia contributors. (2025a, July 13). Peripheral stem cell transplantation. Wikipedia. https://en.wikipedia.org/wiki/Peripheral_stem_cell_transplantation
Norooznezhad, A. H., Malek Mohammadi, A., Kamranzadeh Fumani, H., Aminian, P., Jalili, M., Nikbakht, M., Mousavi, S. A., Vaezi, M., Heshmati, F., Mohammadi, S., Alimoghaddam, K., & Ghavamzadeh, A. (2019). Peripheral blood stem cell apheresis in low-weight children: A single centre study. Transfusion and Apheresis Science, 58(3), 300–303. https://doi.org/10.1016/j.transci.2019.04.018
Lie, A. K., & To, L. B. (1997). Peripheral Blood Stem Cells: Transplantation and Beyond. The oncologist, 2(1), 40–49.
Körbling, M., & Freireich, E. J. (2011). Twenty-five years of peripheral blood stem cell transplantation. Blood, 117(24), 6411–6416. https://doi.org/10.1182/blood-2010-12-322214
Mishra, P. C., Seth, T., & Mahapatra, M. (2015). Peripheral blood stem cell transplant in aplastic anemia. Biology of Blood and Marrow Transplantation, 21(2), S38. https://doi.org/10.1016/j.bbmt.2014.11.031
Contact US

Dr. David Greene
MD, PhD, MBA
Dr. David Greene, MD, PhD, MBA, is a pioneering leader in regenerative medicine and healthcare marketing. As a residency and fellowship-trained orthopedic surgeon, Dr. Greene transitioned from clinical practice to become the founder and CEO of R3 Stem Cell and US Lead Network, where he has revolutionized patient care and medical practice growth through innovative therapies and digital marketing strategies. He has authored two influential books on healthcare internet marketing, ranks among the top expert authors globally, and has been featured on the cover of Corporate Vision magazine for his impact on global regenerative therapies. Beyond his professional achievements, Dr. Greene is passionate about education, compassion, and continuous innovation.