Stem Cell Therapy for Peripheral Arterial Disease (PAD)
Written by Dr. David Greene, MD, PhD, MBA on December 13, 2013
The US Leader in Stem Cell Therapy, Now in Mexico. Treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
The US Leader in Stem Cell Therapy, Now in Mexico. Affordable treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
Written by Dr. David Greene, MD, PhD, MBA on December 13, 2013

There are several medical conditions that result in damage in the blood vessels and arteries of the extremities. Damage in these areas can reduce the amount of blood a limb receives, lowering the amount of oxygen available to that limb for use during function. The only current options available to patients with PAD are surgical correction, with the final option of amputation if surgery is not beneficial.
There may be new hope for patients however, as regenerative treatment with stem cell therapy is showing remarkable progress in the repair and growth of new blood vessels.
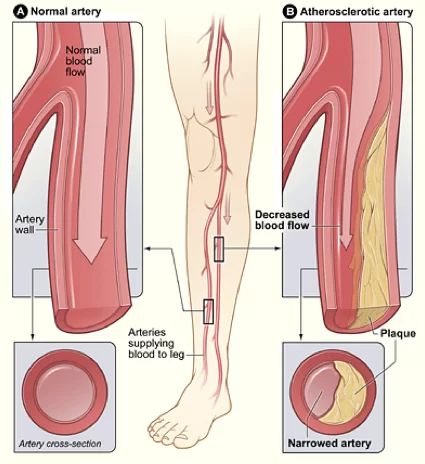
Peripheral arterial disease (PAD) is the combination of these damaging conditions, which include diabetes, Buerger’s disease, and ASO. PSD results in impairment of blood and oxygen flow to the external limbs. After an extended period of time with reduced oxygen, the tissue of these areas becomes damaged.
There is currently only one advisable option available to patients with impaired flow: surgical revascularization in an attempt to return optimal blood flow to the limb. For patients who do not find success with this procedure, amputation of the limb is the last option.
Peripheral arterial disease (PAD) is the combination of these damaging conditions, which include diabetes, Buerger’s disease, and ASO. PSD results in impairment of blood and oxygen flow to the external limbs. After an extended period of time with reduced oxygen, the tissue of these areas becomes damaged.
There is currently only one advisable option available to patients with impaired flow: surgical revascularization in an attempt to return optimal blood flow to the limb. For patients who do not find success with this procedure, amputation of the limb is the last option.
As of this writing, there are two primary approaches in the treatment of PAD through stem cell therapy. The first is the direct harvesting of stem cells from the bone marrow of the patient for use in therapy. Alternatively, a hormone will be administered into the patient to stimulate bone marrow into producing excess stem cells. These will be freely released into the blood stream where they can be collected via routine blood withdrawal.
Regardless of the stem cell collection method used, the stem cells will be isolated and multiplied to increase their numbers. Once a sufficient number of cells are present, they will be transplanted back into the patient. The delivery method of these stem cells may vary, with several different methods currently under testing.

There is substantial evidence that this method of stem cell treatment is sound and that it can potentially produce the desired effect in patients with Peripheral arterial disease (PAD). The best method of collection and re-implantation are still currently under research, which a number of studies currently actively recruiting patients.
Physician First Choice offers stem cell treatments with Board Certified doctors, including treatment for peripheral artery disease. For more information and scheduling, call 888-988-0515.
Contact US