Induced Pluripotent Stem Cells (iPSCs): A New Era in Regenerative Medicine
Written by Dr. David Greene, MD, PhD, MBA on August 8, 2025
The US Leader in Stem Cell Therapy, Now in Mexico. Treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
The US Leader in Stem Cell Therapy, Now in Mexico. Affordable treatments start at $3750 for 25 million stem cells!
Special Promo: Get an additional 25 BILLION Exosomes IV with treatments over 50 million cells!”
Written by Dr. David Greene, MD, PhD, MBA on August 8, 2025
At R3 Stem Cell Mexico, we’re all about bringing the latest and most exciting advancements in medical science to those who need them most. One such advancement, Induced Pluripotent Stem Cells (iPSCs), has been making waves in regenerative medicine, offering the potential to treat conditions that were once thought incurable. But what exactly are iPSCs, and how do they fit into the broader picture of medical progress? Let’s break it down.
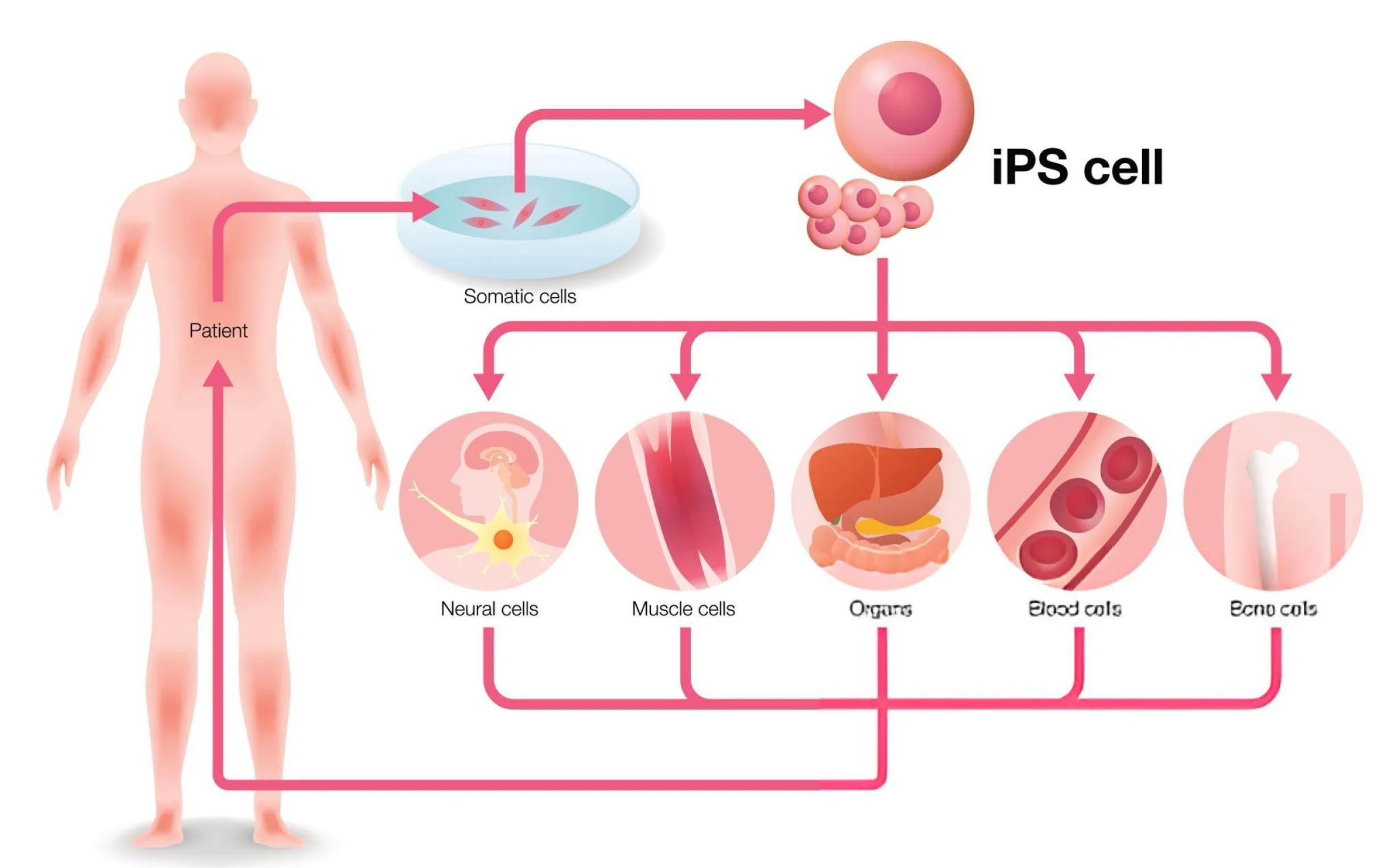
Induced pluripotent stem cells are reprogrammed adult cells—such as skin or blood cells—that have been re-engineered to act like embryonic stem cells. This reprogramming process enables them to differentiate into virtually any type of cell in the body, from nerves to heart cells to muscle tissue.
In other words, iPSCs are versatile. They can become neurons, heart muscle cells, pancreatic beta cells, and much more. This ability to transform into any cell type makes them invaluable for both research and potential therapeutic applications. And perhaps most exciting of all? iPSCs can be derived from the patient’s own cells, which minimizes the risk of immune rejection in therapy.
The discovery of iPSCs dates back to 2006, when Japanese researchers Shinya Yamanaka and Kazutoshi Takahashi at Kyoto University achieved the groundbreaking feat of reprogramming adult cells into pluripotent stem cells. Their work was so influential that they were jointly awarded the 2012 Nobel Prize in Physiology or Medicine alongside Sir John Gurdon, who had previously demonstrated that mature cells could be reprogrammed into more primitive states.
These discoveries set off a scientific revolution, paving the way for iPSCs to become a crucial tool in biomedical research and regenerative medicine.
Creating iPSCs involves introducing specific genes into somatic cells (like skin or blood cells), which effectively “reprograms” them back into a pluripotent state. The key genes responsible for this reprogramming process, often referred to as the Yamanaka factors, include:
These genes play a central role in resetting the cell’s genetic programming, effectively turning back the biological clock. But not all methods of generating iPSCs are the same. Initially, retroviral vectors (viruses) were used to deliver these genes into cells, but this method carries risks due to the possibility of genomic integration. Today, safer non-viral methods are emerging, like the use of synthetic mRNA or protein-based reprogramming, which reduces the risk of insertional mutagenesis—a type of genetic alteration that can lead to unwanted mutations.
The potential uses of iPSCs are truly game-changing. From disease modeling to drug discovery to regenerative medicine, iPSCs open up new possibilities that weren’t possible just a decade ago. Let’s take a closer look at why iPSCs are so valuable:
iPSCs offer a powerful tool for understanding and studying diseases. By reprogramming a patient’s own cells, scientists can create disease models that mirror real-life conditions. For instance, researchers can use iPSCs to study diseases like Parkinson's, Huntington's disease, and diabetes in ways that were previously impossible.
With these models, scientists can investigate how diseases develop, test potential treatments, and explore new therapies—potentially speeding up the process of finding cures.
iPSCs hold the promise of personalized medicine. Since they are derived from a patient’s own cells, they can be used to create patient-specific models for testing drug responses. This could lead to more effective and targeted treatments, reducing the trial-and-error approach that often accompanies traditional drug therapies.
Perhaps one of the most exciting applications of iPSCs is in regenerative medicine. These cells have the ability to generate tissues or even organs, which could be used to repair damaged or diseased tissues. For example, iPSCs have already been used to create cardiomyocytes (heart cells) that function well after being transplanted into mice. In theory, this could one day help treat heart disease, spinal cord injuries, diabetes, and other conditions that currently have no cure.
Let’s do a quick comparison:
Type
Source
Can become other cells?
Common use
Embryonic stem cells
Donated embryos
Yes
Broad research, controversial
Adult stem cells
Bone marrow, fat, etc.
Limited types
Joint repair, immune issues
iPSCs
Your own reprogrammed cells
Yes (like embryonic)
Still emerging, very promising
So, iPSCs give you the flexibility of embryonic cells without the ethical baggage. It’s like getting all the options on the menu without the guilt trip.
While iPSCs are incredibly promising, there are still a few hurdles to overcome. One of the biggest concerns is the tumorigenicity of iPSCs. Just like embryonic stem cells, iPSCs can form teratomas (tumors) when introduced into animals, especially if they are not properly controlled. Research is ongoing to find ways to reduce these risks, such as omitting certain reprogramming factors like c-Myc, which has been linked to tumor formation.
Another challenge is the efficiency of reprogramming, which, although improving, is still far from perfect. In early experiments, the reprogramming efficiency was a mere 0.01–0.1%, though newer techniques have brought this number much higher. That said, there is still work to be done before iPSCs can become a routine therapy in clinical settings.
As of 2020, there were 131 clinical trials involving pluripotent stem cells (PSCs), with approximately 74.8% of these involving induced pluripotent stem cells (iPSCs). These trials are being conducted globally, with the USA, France, China, and Japan being major contributors. The majority of these trials are observational, focusing on disease modeling and cell generation. Approximately 22.9% are interventional trials, involving cell transplantation. Over 1,200 patients have been treated with stem cell-based products, including iPSC derivatives, marking a significant milestone in regenerative medicine.
One of the most exciting applications of iPSCs is in drug discovery. Traditionally, testing new drugs involves using animal models, but iPSCs offer a more accurate, human-based approach to studying how new treatments affect the body.
With iPSCs, researchers can create models of human diseases using patient-specific cells. For example, a patient with Parkinson’s disease could have their own skin cells reprogrammed into iPSCs, and then those iPSCs could be differentiated into dopaminergic neurons (the kind of cells that are damaged in Parkinson’s). This model would then be used to screen drugs to see which ones might be effective for that patient specifically.
This ability to personalize drug testing opens up a world of possibilities, reducing the trial-and-error approach currently used in drug development. It also enables researchers to study diseases at a cellular level, offering a much clearer picture of how diseases progress and how new therapies can intervene.
Imagine being able to receive a treatment tailored just for you—one that takes your unique genetic makeup into account and is designed to work with your body’s individual needs. That’s the promise of personalized medicine powered by iPSCs.
Here’s how it works: by creating iPSCs from a patient’s own cells, doctors can study the genetic and biological factors that make that patient unique. This could lead to therapies that are far more effective because they are designed specifically for the patient’s biology. In fact, this is already happening in clinical trials around the world. Patient-matched iPSCs have been used in research on immune diseases, cancer, and neurodegenerative conditions.
By using a patient’s own iPSCs, doctors can also reduce the risks associated with immune rejection, which is a major concern in cell-based therapies. This means that patients will be able to receive stem cell treatments that are safe, effective, and personalized.
Perhaps the most promising application of iPSCs lies in regenerative medicine, the field focused on repairing or replacing damaged tissues and organs. iPSCs have the potential to regenerate entire organs, offering the possibility of treating chronic diseases and life-threatening conditions that currently have no cure.
For example, heart disease and spinal cord injuries are areas where iPSCs could make a big difference. iPSC-derived cardiomyocytes (heart cells) have been transplanted into rodent models of heart disease, and the results have shown impressive survival and functionality. Parkinson’s disease has also been studied using iPSC-derived neurons, with encouraging results in rodent models showing that these neurons could help repair the brain.
What makes this so exciting is that iPSCs can be generated from a patient’s own cells, meaning tissues or organs could potentially be grown to match that patient’s genetic profile, making them a perfect match and eliminating the risk of immune rejection.
The potential to grow whole organs from iPSCs is not science fiction—it's becoming a reality. Researchers are already working on generating functional organs like livers, hearts, and even kidneys using iPSCs. While we’re still a long way from having lab-grown organs ready for human transplantation, progress is being made.
In fact, iPSC-derived liver cells have been used in experimental models to help treat liver disease, and iPSC-derived heart cells have been tested in models of myocardial infarction (heart attack). The idea of growing fully functional organs in the lab to replace damaged or diseased ones could be life-changing for patients on organ transplant lists, where donor organ shortages are a major issue.
iPSCs are transforming the way we study diseases. Unlike traditional animal models, iPSCs allow scientists to create human disease models that reflect the exact genetic makeup of the individual patient. This is particularly important for diseases that are genetically complex or involve specific mutations, such as Parkinson's, Alzheimer’s, ALS, and diabetes.
With patient-specific iPSCs, researchers can investigate the causes of disease, track disease progression, and test potential treatments—all using real human cells. This approach provides a level of precision and accuracy that animal models simply can’t offer, enabling better-targeted therapies and improving our understanding of complex diseases.
While iPSCs hold incredible promise, they are not without their challenges. Some of the main concerns include:
Tumorigenicity: iPSCs, like embryonic stem cells, can form tumors (called teratomas) when transplanted. This is primarily due to the reprogramming factors used in their creation, particularly c-Myc, which is an oncogene.
Efficiency: While reprogramming efficiency has improved over the years, it’s still not 100%. Some methods of reprogramming, such as using retroviral vectors, come with risks of genomic insertion, which can lead to unwanted mutations.
Ethical Concerns: Although iPSCs don’t involve embryos, there are still ethical questions around their use, especially when it comes to the genetic manipulation of cells.
That said, the future of iPSCs in medical treatments looks incredibly promising. With continued research and clinical trials, we are one step closer to harnessing the full potential of iPSCs to treat a wide range of diseases and even regenerate lost tissues and organs.
The potential of induced pluripotent stem cells (iPSCs) in medicine is nothing short of remarkable. From disease modeling to personalized treatments and organ regeneration, iPSCs offer hope for conditions that were once thought incurable. At R3 Stem Cell Mexico, we are excited to be a part of this rapidly advancing field and look forward to the many breakthroughs ahead.
As clinical trials continue to unfold, we remain hopeful that the future will bring life-changing therapies powered by iPSCs, opening new doors for patients worldwide. The journey is just beginning, and we’re here to help you stay informed and ready for the breakthroughs that lie ahead.
Contact US

Dr. David Greene
MD, PhD, MBA
Dr. David Greene, MD, PhD, MBA, is a pioneering leader in regenerative medicine and healthcare marketing. As a residency and fellowship-trained orthopedic surgeon, Dr. Greene transitioned from clinical practice to become the founder and CEO of R3 Stem Cell and US Lead Network, where he has revolutionized patient care and medical practice growth through innovative therapies and digital marketing strategies. He has authored two influential books on healthcare internet marketing, ranks among the top expert authors globally, and has been featured on the cover of Corporate Vision magazine for his impact on global regenerative therapies. Beyond his professional achievements, Dr. Greene is passionate about education, compassion, and continuous innovation.